Kenna
Ureilite
Monomict/Unbrecciated
Olivine–pigeoniteLow-Ca clinopyroxene, (Ca,Mg,Fe)SiO3, found as a major mineral in eucrites and shergottites. In order to be considered pigeonite, the clinopyroxene must contain 5 to 20 mol % of calcium (Wo5 - 20). Chondrites of petrologic types 4 and below contain significant low-Ca clinopyroxene. During metamorphism to higher temperatures, all existing
Found February 1972
33° 54′ N., 103° 33.2′ W. A single stone of 10.9 kg was found in Roosevelt County, New Mexico by Ivan ‘Skip’ Wilson on his ranch. It is composed mainly of olivineGroup of silicate minerals, (Mg,Fe)2SiO4, with the compositional endpoints of forsterite (Mg2SiO4) and fayalite (Fe2SiO4). Olivine is commonly found in all chondrites within both the matrix and chondrules, achondrites including most primitive achondrites and some evolved achondrites, in pallasites as large yellow-green crystals (brown when terrestrialized), in the silicate portion Click on Term to Read More and the calcium-poor pyroxeneA class of silicate (SiO3) minerals that form a solid solution between iron and magnesium and can contain up to 50% calcium. Pyroxenes are important rock forming minerals and critical to understanding igneous processes. For more detailed information, please read the Pyroxene Group article found in the Meteoritics & Classification category. Click on Term to Read More, pigeonite, in a black carbon-rich matrixFine grained primary and silicate-rich material in chondrites that surrounds chondrules, refractory inclusions (like CAIs), breccia clasts and other constituents. Click on Term to Read More containing graphiteOpaque form of carbon (C) found in some iron and ordinary chondrites and in ureilite meteorites. Each C atom is bonded to three others in a plane composed of fused hexagonal rings, just like those in aromatic hydrocarbons. The two known forms of graphite, α (hexagonal) and β (rhombohedral), have Click on Term to Read More, lonsdaleiteHexagonal polymorph of carbon (C) that forms from meteoric graphite during impact. The immense heat and stress of the impact transforms the graphite into diamond, but retains graphite's hexagonal crystal lattice (below). Lonsdaleite was first identified from the Canyon Diablo meteorite at Barringer Crater (also known as Meteor Crater) in Click on Term to Read More, and diamondOne of the naturally occurring forms of carbon found in meteorites. Each C atom is bonded through covalent sp3 hydrid orbitals to four others. The strength of the C-C bonds makes diamond the hardest naturally occurring substance (according to the Mohs scale) in terms of resistance to scratching. There are Click on Term to Read More. Melt pockets and secondary veins contain grains of Ca-rich augiteHigh-Ca clinopyroxene, (Ca,Mg,Fe)SiO3, that occurs in many igneous rocks, particularly those of basaltic composition. In order to be considered augite, the clinopyroxene must contain 20 to 45 mol % of calcium (Wo20 - 45). An important and unique Martian meteorite is NWA 8159, that has been classified as an augite basalt. Click on Term to Read More, andesine, K-feldspar, diopside, and chromiteBrownish-black oxide of chromium and iron (Cr-Fe oxide), Cr2FeO4, found in many meteorite groups. Click on Term to Read More.
- high FeO/MgO; <80 mol% fo (e.g., Kenna [Fo~79], Novo Urei, Goalpara, Haverö)
- intermediate FeO/MgO; 80-90 mol% Fo (e.g., Dingo Pup Donga, Hajmah (a), Dyalpur)
- low FeO/MgO; >90 mol% Fo (e.g., Y-74659, LEW 85440, Almahata Sitta #051)
Subsequent studies of a larger sample set indicates that these FeO/MgO ratios might actually represent a continuum. More recently, Goodrich et al. (2002, 2004) have proposed a model to describe the petrogenesis of this meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More group; some details from their model are included here. Their model recognizes three mineralogical types of monomict/unbrecciated ureilites:
- The most numerous type, comprising ~90% of all ureilites, has an olivine–pigeonite composition. These ureilites are augite-depleted, partial melt residues produced through low degrees (<15–30%) of fractional melting at temperatures reaching ~1250°C and occurring over a range of depths, while undergoing various degrees of reductionOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More through smelting. Both the basaltic melts and the molten metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More were extracted through veins and dikes, likely propelled off of the planetesimal by the pressure of smelting-derived CO+CO2 gas. Alternatively, an oblique collision with a smaller asteroid could have resulted in the removal of the basaltic mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More, with the mixing of olivines in the regolithMixture of unconsolidated rocky fragments, soil, dust and other fine granular particles blanketing the surface of a body lacking an atmosphere. Regolith is the product of "gardening" by repeated meteorite impacts, and thermal processes (such as repeated heating and cooling cycles). Click on Term to Read More occurring through subsequent impact gardening (Downes et al., 2008). This ureilite type has FeO/MgO ratios in the range of Fo~76–87, and it features Δ17O values of –0.5 to –1.0.
- Of the remaining ~10% of ureilites, an olivine–orthopyroxeneOrthorhombic, low-Ca pyroxene common in chondrites. Its compositional range runs from all Mg-rich enstatite, MgSiO3 to Fe-rich ferrosilite, FeSiO3. These end-members form an almost complete solid solution where Mg2+ substitutes for Fe2+ up to about 90 mol. % and Ca substitutes no more than ~5 mol. % (higher Ca2+ contents occur Click on Term to Read More composition has been found in a few samples (e.g., LEW 85440 and pairings, Y-74659, Y-791538, EET 87517, MET 01085, and EET 96262 and pairings). These ureilites are residues formed from rare, fractionated, late-stage melt pockets in close association with olivine–pigeonite ureilitic residues at temperatures reaching ~1275°C. Smelting/reduction likely occurred both at the source and at lower pressures as the melt ascended through shallower depths; some pigeonite may still remain. This ureilite type is highly magnesian with FeO/MgO ratios in the range of Fo~86–92, and it features Δ17O values of –2.0 to –2.5.
- The remainder of the monomict/unbrecciated ureilites have an olivine–augite (augite-bearing) composition, and only a small number of examples have been described thus far (e.g., ALH 82130 and pairings, EET 96293 and pairings, FRO 90054 and pairings, HaH 064, Hughes 009, LEW 88774, META78008, and Y-74130. In this ureilite type, pigeonite has been replaced by augite (~5–30%), while orthopyroxene also is typically present (~29–56%), usually having poikilitic textures. A poikilitic or bimodal texture is present in many of these ureilites, exhibiting large orthopyroxene oikocrysts that formed from a late melt phase enclosing olivine–augite textural zones; however, a few have a typical ureilite texture. Members of this type are probably cumulates or paracumulates that formed from melts originating at great depths, crystallizing after ascent to shallower depths and experiencing some degree of smelting/reduction. They span a broad range of ureilite FeO/MgO ratios of Fo76–95.
Paradoxically, the pressure-controlled smelting model shows that ureilites in our collections are biased in sampling only a thin layer at intermediate depth within the pre-impact parent bodyThe body from which a meteorite or meteoroid was derived prior to its ejection. Some parent bodies were destroyed early in the formation of our Solar System, while others like the asteroid 4-Vesta and Mars are still observable today. Click on Term to Read More, which exhibits a spike at Fo76–81 (Warren, 2012). A model of gasless anatexis (magmatism) is consistent with high internal pressures, suggesting a relatively large pre-impact parent body of at least 400 km in diameter. Efforts continue in ernest to constrain the numerous parameters of the ureilite petrogenetic history by employing numerical modeling (e.g., Michel et al., 2013 #1300) including the following:
- smelting vs. nebular inheritance of olivine Fo variation among ureilites
- pre-impact structure of ureilite parent body (e.g., (i) metallic coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More vs. no core (ii) molten vs. partially molten vs. solid vs. solid w/molten layer)
- pre-impact radius of ureilite parent body
- catastrophic vs. sub-catastrophic breakup and reassembly
- properties of the impacting projectile (e.g., size, speed, impact angle)
- formation of one or more ureilite daughter bodies vs. re-acreation of rubble layer on the original parent body
- original depth on the parent body (within temperature and pressure constraints) from which material was derived to construct the theorized daughter body(ies)
Kenna is a member of Berkley’s least reducedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More subgroup I, being FeO-rich with a high fayalitePure* iron end-member (Fe2SiO4) of the olivine solid solution series and an important mineral in meteorites. When iron (Fe) is completely substituted by magnesium, it yields the the pure Mg-olivine end-member, forsterite (Mg2SiO4). The various Fe and Mg substitutions between these two end-members are described based on their forsteritic (Fo) Click on Term to Read More content (Fo~79). It is proposed that members of this subgroup formed early at the deepest layers, and are associated with the highest C content. It also belongs to Goodrich’s subgroup 1, having a composition consisting primarily of olivine and pigeonite. Goodrich et al. (2002; 2004) described a stratification of the ureilite precursor material consistent with the existence of both magnesian (shallower) and ferroan (deeper) ureilites. This stratification is thought to have been originally established through pre-igneous aqueous flows allowing for the heterogeneous distribution of unfractionated O-isotopes, as well as the concentration of other mineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More elements such as Ca.
The precursor material of ureilites had a bulk composition similar to that of carbonaceous chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More, including O-isotopic compositions having strongly negative Δ17O plotting along the carbonaceous chondriteCarbonaceous chondrites represent the most primitive rock samples of our solar system. This rare (less than 5% of all meteorite falls) class of meteorites are a time capsule from the earliest days in the formation of our solar system. They are divided into the following compositional groups that, other than Click on Term to Read More anhydrous mineral (CCAM) trend line. In addition, it contains a relatively high C content (6–7 wt%). In particular, characteristics very similar to precursor ureilite material are found among the CV-group chondrites, such as bulk FeO/MgO ratios equivilant to Fo~62. However, noteworthy differences exist between the ureilite precursor material and CV-like material; e.g., the former has a higher, superchondritic Ca/Al ratio (~2.5 × CI) compared to CV-like material, and depending on the model used, the ureilite precursor material may have had an increased Si/Mg ratio comparable to that found in ordinary chondrites as well as a very low alkali content (Goodrich et al., 2007). Modelling to determine the precursor end-member components from which the ureilite parent body was formed was pursued by Rai et al. (2015). They searched for combinations of components which when combined would exhibit the elemental ratios and oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More isotopeOne of two or more atoms with the same atomic number (Z), but different mass (A). For example, hydrogen has three isotopes: 1H, 2H (deuterium), and 3H (tritium). Different isotopes of a given element have different numbers of neutrons in the nucleus. Click on Term to Read More signature of known ureilites. They found that a combination of three end-member components comprising Fe-rich and Fe-poor chondrulesRoughly spherical aggregate of coarse crystals formed from the rapid cooling and solidification of a melt at ~1400 ° C. Large numbers of chondrules are found in all chondrites except for the CI group of carbonaceous chondrites. Chondrules are typically 0.5-2 mm in diameter and are usually composed of olivine Click on Term to Read More plus either CI-type or CM-type chondritic material best matched the range of values found in ureilites. Carbonaceous chondrites are too FeO-rich and SiO2-poor to represent ureilite precursor material, and ureilite feldspathic glass is not a consistent product of such an origin (Warren, 2011). Moreover, ε48Ca is significantly different between ureilites and carbonaceous chondrites (Chen et al., 2011). In the same way, a plot of ε50Ti vs. ε54Cr and Δ17O vs. ε54Cr finds ureilites actually cluster far from, and in an orthogonal direction from, the carbonaceous chondriteChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More trend. Moreover, ureilites have lower ε62Ni and far lower ε50Ti and ε54Cr than any known carbonaceous chondrite. Based on isotopic and compositional parameters, it was concluded that an extension of the carbonaceous chondrite trend to incorporate the ureilites was not supported, and a predominantly non-carbonaceous chondritic precursor for ureilites is recognized as more reasonable (Warren, 2011). Based on REEOften abbreviated as “REE”, these 16 elements include (preceded by their atomic numbers): 21 scandium (Sc), 39 Yttrium (Y) and the 14 elements that comprise the lanthanides excluding 61 Promethium, an extremely rare and radioactive element. These elements show closely related geochemical behaviors associated with their filled 4f atomic orbital. Click on Term to Read More correlation trends and their abundances in ureilite augites, Huang et al. (2009) concluded that ureilites were formed through partial meltingAn igneous process whereby rocks melt and the resulting magma is comprised of the remaining partially melted rock (sometimes called restite) and a liquid whose composition differs from the original rock. Partial melting occurs because nearly all rocks are made up of different minerals, each of which has a different melting Click on Term to Read More of precursor material that is consistent with depleted spinelMg-Al oxide, MgAl2O4, found in CAIs. peridotite with a 5% clinopyroxene component. Warren (2011) determined that the isotope signatures of Δ17O, ε54Cr, ε50Ti, and ε62Ni can be utilized to resolve carbonaceous from non-carbonaceous meteorites; the carbonaceous meteorites have positive values for all of these elements, while the non-carbonaceous meteorites have negative values. An example coupled Δ17O vs. ε54Cr diagram is shown below to demonstrate the separation between carbonaceous and non-carbonaceous meteorites. Ureilites plot in the non-carbonaceous field furthest removed from the carbonaceous field.
Diagram credit: Sanders et al., MAPS, vol. 52, #4, p. 695 (2017)
‘Origin of mass-independent oxygen isotope variation among ureilites: Clues from chondrites and primitive achondrites’
(http://dx.doi.org/10.1111/maps.12820) In calculating the required Ca/Al ratio from which a precursor magmaMolten silicate (rock) beneath the surface of a planetary body or moon. When it reaches the surface, magma is called lava. Click on Term to Read More would produce pigeonite-type residues, it was determined that this ratio was substantially higher than chondritic levels (2.5–3 × CI), and therefore, a parent magma depleted in plagioclaseAlso referred to as the plagioclase feldspar series. Plagioclase is a common rock-forming series of feldspar minerals containing a continuous solid solution of calcium and sodium: (Na1-x,Cax)(Alx+1,Si1-x)Si2O8 where x = 0 to 1. The Ca-rich end-member is called anorthite (pure anorthite has formula: CaAl2Si2O8) and the Na-rich end-member is albite Click on Term to Read More would be a requirement; e.g., segregation during melting and loss through explosive volcanism. Alternatively, the Ca could have been concentrated during aqueous alteration and dehydration phases to form heterogeneous regions. Still, another process described as fractional smelting has been invoked by Singletary and Grove (2006) to account for the high Ca/Al ratios in ureilites (ave. 4.2 × CI, but as high as 14.5 × CI). They suggest that an olivine+melt+carbon+metal source underwent decompression smelting, perhaps promoted by cracks, which lead to an enrichment in Ca compared to Al as temperatures cooled below ~1240°C. At the same time, this process resulted in an enrichment of chromite along with high Mg# silicateThe most abundant group of minerals in Earth's crust, the structure of silicates are dominated by the silica tetrahedron, SiO44-, with metal ions occurring between tetrahedra). The mesodesmic bonds of the silicon tetrahedron allow extensive polymerization and silicates are classified according to the amount of linking that occurs between the residues. An alternative model for the existence of superchondritic Ca/Al ratios in ureilites was proposed by Goodrich et al. (2007). They determined that pigeonite-bearing residues with the highest measured Mg# could be produced with a Ca/Al ratio of ~2.5 × CI. They proposed a disequilibrium model in which melt was rapidly extracted through a complex vein and dikePlanar, blade-like, intrusive igneous body that cuts across preexisting layers usually at a high-angle to near-vertical orientation. By definition, a dike is always younger than the rocks that contain it. Terrestrial dikes are typically 0.5 to 3 m wide and extend for a few 10s of kilometers. Click on Term to Read More network faster than it could be replenished, on a time scale of weeks to a year—a process they refer to as fractional melt extraction. This rapid-to-immediate extraction process only allows for a very low diffusivity of O-isotopes, Ca, REE, and highly siderophile trace elements from the silicate residue to the melt phase, resulting in the preservation of heterogeneity and certain chondritic ratios within the thermally processed ureilites—features which had been historically considered paradoxical (Wilson and Goodrich, 2012). This model reasonably addresses the concerns propounded by Mittlefehldt et al. (2005) as to why there was an increase in the abundance of siderophile elements like Ni and Ir among the most magnesian ureilites at the same time that the smelting-produced Fe-metal host phase was removed. In their study of Re–Os isotope systematics of ureilites, Rankenburg et al. (2007) showed that a similar Os-isotopic distribution exists between ureilites and carbonaceous chondrites, particularly the CM, CV, CO, and CR groups, consistent with their formation in a common nebular region. The constant Os-isotopic ratios in ureilites demonstrate that there was a lack of fractionationConcentration or separation of one mineral, element, or isotope from an initially homogeneous system. Fractionation can occur as a mass-dependent or mass-independent process. Click on Term to Read More of Re from Os on the ureilite PB, similar to what is indicated in carbonaceous chondrites. They also found that a metal component must have remained in the ureilite PB during its melt phase in order to stabilize Re against its extraction through the melt. However, in a rapid fractional melt extraction model, molten metal is also thought to be removed prior to any significant diffusionMovement of particles from higher chemical potential to lower chemical potential (chemical potential can in most cases of diffusion be represented by a change in concentration). Diffusion, the spontaneous spreading of matter (particles), heat, or momentum, is one type of transport phenomena. Because diffusion is thermally activated, coefficients for diffusion Click on Term to Read More of highly siderophile elements from the silicate residue. Paradoxically, the theory that the carbonaceous chondrite parent asteroids did not undergo fractionation or differentiationA process by which a generally homogeneous chondritic body containing mostly metal, silicates and sulfides will melt and form distinct (differentiated) layers of different densities. When the melting process continues for a long enough period of time, the once chondritic body will re-partition into layers of different composition including Click on Term to Read More runs contrary to the model of Bunch et al. (2005), who utilized O-isotopic data to reconstruct the CV and CR parent bodies (see the Allende page for details). The disequilibrium model of fractional melt extraction does provide a means for the extraction of large quantities of smelting-produced metal. Petrogenetic constraints based on ureilite mineralogy and a CV-like precursor require a loss of ~15–24 wt% FeO through either core migration or explosive volcanism of reduced molten metal. Rapidly formed CO+CO2 gas bubbles could have assisted in the ascent of S-rich liquid metal upwards to the surface and into space. Singletary and Grove (2006) proposed that the high pressures generated from smelting-produced CO+CO2 gas may have had no escape pathway and promoted a disruption of the ureilite parent body. An alternative disruption mechanism, proposed by Downes et al (2008), invokes an oblique impact between the proto-ureilite body and a smaller planetesimal resulting in the loss of its outer layers coupled with asteroid-wide pressure-release reduction processes. Studies of the REE contents of Kenna are most consistent with an incremental batch melting process. However, in their analysis of the conditions that prevailed during the partial melting phase, including a calculation of the predicted Sm content in ureilites, Warren et al. (2006) became convinced that a continuous partial melting process is the best fit to the data, and that a silicate melt porosityThe volume percentage of a rock that consists of void space. Vesicular porosity is a type of porosity resulting from the presence of vesicles, or gas bubbles, in igneous rock such as the pumice presented here. Vesicular porosity is very rare in meteorites and is often associated with slag, one Click on Term to Read More of ~5–10% was probably established during the partial melt phase. This permitted the loss of a sulfur melt component (and other siderophile fractionations) into the core, while sustaining the explosive volcanism of a significant Al-bearing basaltic component. Rankenburg et al. (2008) showed that the Fe deficiency that exists in ureilites, compared to their probable carbonaceous chondrite-like precursor, is proportional to the amount of S presumed to be initially present, which together would be utilized to form an FeS total metallic melt composition. They also suggested a theory for how the highly siderophile elementLiterally, "iron-loving" element that tends to be concentrated in Fe-Ni metal rather than in silicate; these are Fe, Co, Ni, Mo, Re, Au, and PGE. These elements are relatively common in undifferentiated meteorites, and, in differentiated asteroids and planets, are found in the metal-rich cores and, consequently, extremely rare on depletion of ureilites could have been established, which they propose occurred through the grain-scale mixing of two separate components, each having different refractory HSE (highly siderophile elementSubstance composed of atoms, each of which has the same atomic number (Z) and chemical properties. The chemical properties of an element are determined by the arrangement of the electrons in the various shells (specified by their quantum number) that surround the nucleus. In a neutral atom, the number of Click on Term to Read More) and HSE abundances, during late brecciationThe formation of a breccia through a process by which rock fragments of of various types are recemented or fused together. Click on Term to Read More processes. An early (0.5 m.y. after CAIsSub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More) formation of the ureilite parent body would result in a maximum degree of partial melting of ~30% (Wilson and Goodrich, 2012). An early and rapid phase of silicate melt extraction would result in the removal of radiogenic 26Al, thus preventing further melting of the UPB while retaining its heterogeneous nature. In the course of their studies, Wilson and Goodrich (2012) reconsidered the commonly accepted magma oceanCompletely molten surfaces of terrestrial planets or moons that formed soon after accretion. Samples returned by the Apollo missions provide evidence of a lunar magma ocean, crystallization of which produced a stratified Moon with a low-density crust formed by accumulation of the mineral plagioclase overlying a higher density mantle of Click on Term to Read More model and rejected it in favor of a model in which melt accumulated in massive intrusions at the base of the lithosphereRigid outer layer of a planet. The base of the lithopshere is defined by the temperature at which the brittle/ductile transition occurs in the mantle. Click on Term to Read More. The following model for the thermal and melt extraction history of the UPB is rendered from Fractional melting and smelting on the ureilite parent body, Goodrich et al. (2007), and from Thermal Evolution and Physics of Melt Extraction on the Ureilte Parent Body, Wilson et al. (2008):
Evidence shows that the ureilite parent asteroid must have accreted from an ~80:20 mixture of silicates and ice early in Solar SystemThe Sun and set of objects orbiting around it including planets and their moons and rings, asteroids, comets, and meteoroids. history ~0.55 m.y. after CAISub-millimeter to centimeter-sized amorphous objects found typically in carbonaceous chondrites and ranging in color from white to greyish white and even light pink. CAIs have occasionally been found in ordinary chondrites, such as the L3.00 chondrite, NWA 8276 (Sara Russell, 2016). CAIs are also known as refractory inclusions since they Click on Term to Read More formation, and that it experienced rapid melting primarily from the heat of 26Al decay. Production of an abundant volume of melt was initiated by ~1 m.y. after CAI formation as temperatures reached 1050°C, and this melt production continued by progressively declining degrees over the next ~4 m.y., at which time an overall degree of melting of 30% was achieved.
Their model demonstrates that during the early stage of radiogenic heating, as the degree of melting reached 0.15%, the melt was rapidly buoyed upwards through an interconnected network of veins and fractures, these thought to be largely created during the previous hydrationReaction of a substance with water. Click on Term to Read More and dehydration phases on the asteroid. The size of the veins constituting this hierarchical network ranged from 1 mm at grain boundaries at the melt initiation depths, to sizes of over 10 km as the majority of the melt was efficiently lost into space through dikes during episodic explosive volcanism. The speed of the rising melt was bolstered by high volumes of smelting-produced CO+CO2 gas to easily exceed the escape velocityVelocity that an object needs to escape the primary gravitational influence of a more massive object: where, m = the object's mass, r = distance from object's center, and G = gravitational constant of the larger object. Click on Term to Read More. The entire journey of this melt through the network from grain boundary until extraction from the UPB is calculated to have taken a month at its peak, or only slightly longer thereafter. However, ~15–25% of the total melt volume is expected to have been retained within an extensive asteroid-wide systemDefinable part of the universe that can be open, closed, or isolated. An open system exchanges both matter and energy with its surroundings. A closed system can only exchange energy with its surroundings; it has walls through which heat can pass. An isolated system cannot exchange energy or matter with of perhaps five sill-like intrusions located ~7 km beneath the cold, unmelted outer crustOutermost layer of a differentiated planet, asteroid or moon, usually consisting of silicate rock and extending no more than 10s of km from the surface. The term is also applied to icy bodies, in which case it is composed of ices, frozen gases, and accumulated meteoritic material. On Earth, the Click on Term to Read More, each reservoir extending ~4.7 km × ~144 km. It was shown that a large percentage of this sill component (~76%) likely represents melt from deeper, gas-poor, more ferroan source regions. Such a source region would be consistent with a scenario for the origin of the feldspathic clasts found in polymict ureilites, given their formation ~1 m.y. after CAIs and their accumulation into a near-surface intrusion, where they underwent slow cooling and closure over the next ~4 m.y. Consistent with the large sampling of ureilites studied, it was calculated that these rocks represent strataOriginally horizontal layers of rock. located at depths of between ~10 km and ~50 km, corresponding to pressures of 3 MPa (most magnesian) and 10 MPa (most ferroan), respectively, on an asteroid ~100 km in radius (arguably the radius was between ~20 km and ~475 km). Pressures at its center are estimated to have been ~15.2–26.6 MPa. Melting and smelting are initiated at different times depending on depth. At the shallowest levels where pressures are lowest, ~3 MPa, melting and smelting occur simultaneously as temperatures reach ~1060°C, producing pigeonite residues corresponding to an increase in Mg# from ~62 to 86. This is followed by orthopyroxene production resulting in further progressive increases in Mg# up to ~91, at which point melting and smelting institute pigeonite production again. At increasing depths, smelting begins progressively later than the onset of melting. At the greatest depths inferred for ureilites, corresponding to ~10 MPa of pressure, smelting begins after a temperature of 1200°C is reached, at a point when 21% partial melting has occurred. The production of pigeonite residues is initiated as augite is successively removed through the melt phase. Complete removal of augite occurs at ~30% melting, having attained a molar Fo content of ~76; orthopyroxene production begins thereafter. If ureilite samples should be found from even greater depths, corresponding to pressures greater than ~12.5 MPa, it is predicted that they will be composed entirely of augite and that no smelting will have occurred. The metallic iron that was produced as an end product of the smelting process is believed to have settled into a central core with a radius of ~41 km.A scenario proposed by Goodrich <et al. (2004, 2007) calls for impact disruption of a >200 km diameter proto-ureilite asteroid and its reassembly into one or more daughter objects from which the ureilites were ultimately derived. In support of this model is the non-regolith brecciaWork in Progress ... A rock that is a mechanical mixture of different minerals and/or rock fragments (clasts). A breccia may also be distinguished by the origin of its clasts: (monomict breccia: monogenetic or monolithologic, and polymict breccia: polygenetic or polylithologic). The proportions of these fragments within the unbrecciated material Click on Term to Read More FRO 93008, which contains adjacent fragments of all three ‘Goodrich-model’ lithologies, consistent with the reassembly of a disrupted body. Evidence supporting the early disruption and reassembly of a single ureilite parent body was presented by Downes et al. (2008).
It has been generally accepted that the microscopic diamonds and lonsdaleite found in Kenna and other ureilites were formed by the medium-level impact-shock forces that liberated this meteorite from its parent body. The correlation that exists between the carbonElement commonly found in meteorites, it occurs in several structural forms (polymorphs). All polymorphs are shown to the left with * indicating that it been found in meteorites and impact structures: a. diamond*; b. graphite*; c. lonsdalite*; d. buckminsterfullerene* (C60); e. C540; f. C70; g. amorphous carbon; h. carbon nanotube*. Click on Term to Read More and O-isotopic compositions among the ureilite groups implies that the carbon was indigenous to the source rocks and was not introduced later through impact-melt injection. Examinations of the graphite/diamond relationships found in Kenna and other ureilites using x-ray diffractionAnalytical technique used to determine the structures of crystalline solids. A monochromatic beam of X-rays (usually Cu-Kα) is diffracted off repeating planes of atoms in crystalline samples to produce a diffraction pattern. Through analysis of the diffraction pattern, atomic structures can often be determined. techniques have revealed the presence of compressed graphite. Compressed graphite has been known to occur experimentally under high pressures and temperatures as part of the phase transition to diamond. Furthermore, graphite can be converted to diamond at much lower pressures (above 5.5 GPa) at high temperatures (1400°C) when in the presence of a molten metal transformation catalystChemicals that are not consumed in a reaction, but, which speed up the reaction rate. Catalysts aid to form a transition state which is lower in energy than the transition state without the catalyst (essentially decreasing activation energy). Since the barrier to the reaction is lower, the reaction rate increases Click on Term to Read More. Consistent with this idea is the fact that kamaciteMore common than taenite, both taenite and kamacite are Ni-Fe alloys found in iron meteorites. Kamacite, α-(Fe,Ni), contains 4-7.5 wt% Ni, and forms large body-centered cubic crystals that appear like broad bands or beam-like structures on the etched surface of a meteorite; its name is derived from the Greek word Click on Term to Read More is found in association with all diamond phases but in none of the graphite phases. This research provides solid evidence for the high-pressure catalytic conversion of graphite to diamond in ureilites resulting from an impact event. In their study of primary and polycrystalline secondary graphite phases present in the diamond-bearing, main group ureilite UAE 001, Hezel et al. (2008) concluded that the evidence favors the formation of diamonds in this and other ureilites through shock forces of varying degree and duration. They argued that a prolonged shock duration could be the cause of the smaller FWHM (full width at half maximum) values that are observed in a subset of the Raman bands of meteoritic diamond. They found that the small FWHM values reflect the conversion to a well-ordered state, and are only coincidentally similar to the FWHM values obtained for diamonds formed through a chemical vapor depositionMethod for growing solids in which a gaseous precursor (containing fragments of the desired solid) is decomposed and deposited onto a desired surface. Chemical Vapor Deposition (CVD) is one of the most powerful synthetic methods in material science due to its remarkable flexibility. A variety of surfaces can be coated, Click on Term to Read More (CVD) process. Moreover, they found that the large abundance of secondary graphite coexisting with diamond was inconsistent with CVD. In a separate study, Guillou et al (2009) concluded that a quick shock event is the most reasonable formation process given their observation of diamond inclusions within aligned precursor graphitic structures, a feature inconsistent with a diamond graphitization origin. In the least-shocked, diamond-free ureilite, ALH 78019, the absence of primordial noble gasesElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More in graphite, along with a heavy N-isotopic signature in graphite, was found to be inconsistent with the theory that graphite was a precursor to nanodiamond formed by in situ shock conversion processes (Rai, et al., 2002). Utilizing the ureilite NWA 4742, Guillou et al (2009) studied this paradox in which graphite precursor material is depleted in noble gases, while the nanodiamonds into which it was transformed are noble gas-rich. Their investigation led to a proposal that a mixture of two diamond populations is present; i.e., an early population of unknown origin which contains noble gases, and a later population that was formed by shocked graphite depleted in noble gases. They further suggest that the presence of a noble gas-containing graphitic phase surrounding some nanodiamonds could be the result of back-transformation of the early population of diamonds under conditions of slow cooling following a late shock event. To differentiate between the two competing scenarios for diamond formation on the ureilite parent body, i.e., impact shock vs. chemical vapor deposition (CVD), Nagashima et al. (2012) utilized micro-Raman spectroscopyTechnique of splitting electromagnetic radiation (light) into its constituent wavelengths (a spectrum), in much the same way as a prism splits light into a rainbow of colors. Spectra are not smooth but punctuated by 'lines' of absorption or emission caused by interaction with matter. The energy levels of electrons in to study of carbonaceous material in a number of ureilite samples. The resulting spectral data obtained for the major parameters for diamond (peak position, band intensities, and full width at half maximum [FWHM]) were a better match to diamond produced under CVD rather than shock pressure. Moreover, they demonstrated that there was no correlation of the diamond:graphite ratio to the shock levelA petrographic assessment, using features observed in minerals grains, of the degree to which a meteorite has undergone shock metamorphism. The highest stage observed in 25% of the indicator grains is used to determine the stage. Also called "shock level". Click on Term to Read More, and found the noble gasElement occurring in the right-most column of the periodic table; also called "inert" gases. In these gases, the outer electron shell is completely filled, making them very unreactive. Click on Term to Read More and N-isotopic compositions of graphite, amorphousMaterial without the regular, ordered structure of crystalline solids. Amorphous substances, like glass, lack a definite repeating pattern in their atomic structures (crystallinity). There may be small regions of order, but, overall there is disorder. Click on Term to Read More carbon, and diamond to be in accordance with the CVD model, but not with the shock model. Their results suggest a scenario of chemical deposition of graphite, amorphous carbon, and diamond directly onto high-temperature condensates in the primitive solar nebulaThe primitive gas and dust cloud around the Sun from which planetary materials formed., with the formation of each phase being associated with specific variations in CH4:H2 ratios commensurate with temperature and pressure changes. The migration of carbonaceous material to silicate grain contacts, as well as the occurrence of compressed graphite in conjunction with diamond, was the result of later shock events on the ureilite parent body. All ureilites show evidence of having been rapidly cooled (~2–6°C/hr) through the range of ~1100°C to 650°C, and show signs of a sudden pressure drop that initiated smelting reactions between silicates and graphite to produce reduction rims on olivine and pyroxene grains. This extremely rapid cooling and pressure drop led Herrin et al. (2010) to suggest that the catastrophic disruption of the single ureilite parent body resulted in a cloud of innumerable, small, 2nd-generation bodies having sizes of tens of meters or smaller, which were subsequently reassembled. The common presence of these P–T characteristics in diverse types of ureilites supports the view that they all originated on a common UPB (Warren, 2012). Polymict ureilites contain components with a broad range of Mg# and reduced rims normally found in monomict clasts. This is consistent with an origin on a newly assembled parent body. In another example, the olivine compositions within a single thin sectionThin slice or rock, usually 30 µm thick. Thin sections are used to study rocks with a petrographic microscope. of polymict ureilite EET 87720 was found to span the entire range of olivine compositions recorded for unbrecciated ureilites, and the Mg# distribution is nearly identical to that of unbrecciated ureilites, factors which demonstrate a common origin for all ureilites (Downes and Mittlefehldt, 2006). Furthermore, the wide variety of xenolithic clasts from numerous unrelated parent bodies found within the Almahata Sitta ureilite fallMeteorite seen to fall. Such meteorites are usually collected soon after falling and are not affected by terrestrial weathering (Weathering = 0). Beginning in 2014 (date needs confirmation), the NomComm adopted the use of the terms "probable fall" and "confirmed fall" to provide better insight into the meteorite's history. If Click on Term to Read More supports the scenario of a collisionally-disrupted and reassembled ureilite parent body. Four stages of reduction are now recognized to represent the continuum of the reduction process that occurred on the ureilite parent body. A reduction grade sequence from R1 to R4 reflecting an increased conversion of graphite to metal has been demonstrated by investigators from Northern Arizona University (J. Wittke and T. Bunch) and Kingsborough Community College (C. Goodrich), and they have developed the following classification parameter:| R1 | R2 | R3 | R4 | |
|---|---|---|---|---|
| Graphite/metal (vol%) | >10 | 10–1 | 1 | 0 |
| Rim thickness of reduced olivine | <15 µm | 15–50 µm | <50 vol% of olivine | >50 vol% of olivine |
| Degree of hardness | soft | medium | very hard | extreme |
| Diamonds | none | few | irregular distribution | abundant |
Based on textural studies, a number of distinct types of ureilite metal have been identified by Goodrich et al. (2009). Primary metal exists along grain boundaries and in association with C, P, and S in melt spherules within low-Ca pyroxene (mostly pigeonite), both sites probably existing in a complementary solid–liquid arrangement. Secondary metal occurs as a result of reduction processes in olivine, as inclusions in graphite, and as sub-micron-sized trails produced by shock mobilization. Reduction metal and graphite inclusionFragment of foreign (xeno-) material enclosed within the primary matrix of a rock or meteorite. Click on Term to Read More metal have been identified in Kenna; however, grain boundary metal in Kenna has been completely oxidizedOxidation and reduction together are called redox (reduction and oxidation) and generally characterized by the transfer of electrons between chemical species, like molecules, atoms or ions, where one species undergoes oxidation, a loss of electrons, while another species undergoes reduction, a gain of electrons. This transfer of electrons between reactants Click on Term to Read More, and no Fe–C–S–P spherules have yet been identified. Chronometers such as Sm–Nd reveal an early differentiation age for ureilites of ~4.56 b.y., while the Mn–Cr chronometer applied to feldspathic clasts gives a similar absolute age. High precision Mg-isotopic analyses by Baker and Bizarro (2005) reveal even older ages comparable to that of CAI formation. The short-lived Hf–W chronometer has been utilized in the determination of a very early differentiation stage for ureilites. Budde et al. (2014) utilized the W-isotopic system to constrain the onset of melting and the extraction of both a silicate and a metallic melt. Corresponding to the lowest calculated initial ε182W values obtained for their ureilite samples (~ –3.25), the onset of melting occurred within the first ~2 m.y. of solar system history. Furthermore, thermal evolution modeling based on an 26Al heat source demonstrated that accretionAccumulation of smaller objects into progressively larger bodies in the solar nebula leading to the eventual formation of asteroids, planetesimals and planets. The earliest accretion of the smallest particles was due to Van der Waals and electromagnetic forces. Further accretion continued by relatively low-velocity collisions of smaller bodies in the Click on Term to Read More of the UPB occurred within the first ~1 m.y. With regard to the higher calculated initial ε182W values (up to ~ –2.8), it can be inferred that distinct metal–silicate segregation events occurred, or alternatively, that a temporal variation existed between extractions of metallic and silicate melts. A study of the Mn–Cr isotopic systematics by Shukolykov and Lugmair (2006) revealed that the Cr isotopes in Kenna equilibrated after all of the 53Mn had decayed, and therefore Kenna formed later than some of the other ureilites. An isotopic disturbance is observed for Kenna 4.1 b.y. ago, and it has a CRE age given by Beard and Swindle (2017) of 24.74 m.y. Rare opal-A (amorphous) has been identified in the polymict ureilite EET 83309, which was likely formed through hydration of amorphous silicaSilicon dioxide, SiO2. in the parent body regolith when water was introduced (Downes et al., 2016). The specimen of Kenna shown above is a 3.5 g partial slice. The photo below is an excellent petrographic thin section micrograph of Kenna, shown courtesy of Peter Marmet.
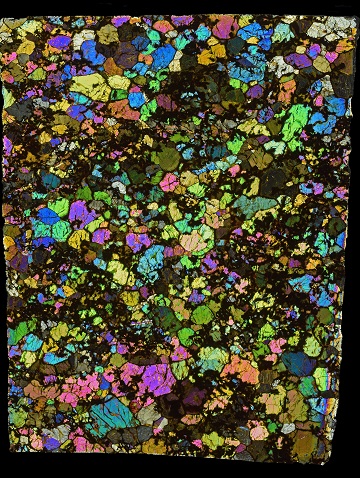
click on image for a magnified view
Photo courtesy of Peter Marmet







