Tazewell
Iron, IAB complex, sLH subgroup
Found 1853
36° 26′ N., 83° 45′ W. A mass of about 60 pounds was plowed up 10 miles west of Tazewell, Tennessee on land owned by Mr. William Rogers. Atmospheric ablationGradual removal of the successive surface layers of a material through various processes. • The gradual removal and loss of meteoritic material by heating and vaporization as the meteoroid experiences frictional melting during its passage through the atmosphere. The resulting plasma ablates the meteor and, in cases where a meteor Click on Term to Read More during entry left an irregularly sculptured shape with large regmaglypts, deep fusion-crusted pits, large holes, and long protuberances.
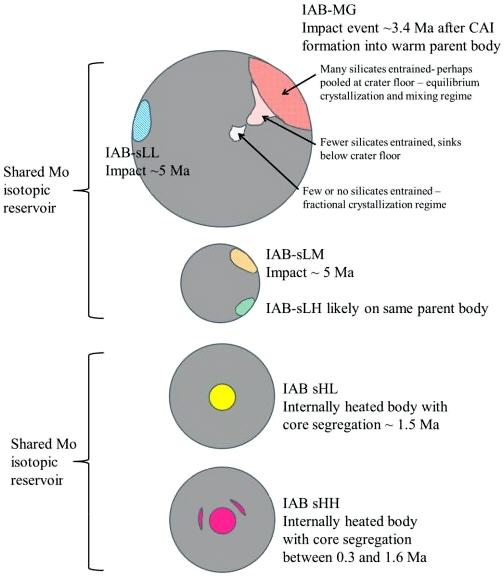
Diagram credit: Worsham et al., Earth and Planetary Science Letters, vol. 467, p. 164 (2017)
‘Characterizing cosmochemical materials with genetic affinities to the Earth: Genetic and chronological diversity within the IAB iron meteoriteIron meteorites consist mostly of metallic iron alloyed with typically between ~5 to ~30 wt% nickel. The main metal phases are kamacite α-(Fe, Ni) and taenite y-(Fe, Ni). Based on their group classification, they may also contain a small weight percentage of one or more of the following minerals: • Click on Term to Read More complex’
(https://doi.org/10.1016/j.epsl.2017.02.044) Dey et al. (2019) employed 17O and ε54Cr values for several irons and their associated silicates/oxides to investigate i) if each iron and its associated phases originated on a common parent body (i.e., an endogenous mixture of coreIn the context of planetary formation, the core is the central region of a large differentiated asteroid, planet or moon and made up of denser materials than the surrounding mantle and crust. For example, the cores of the Earth, the terrestrial planets and differentiated asteroids are rich in metallic iron-nickel. Click on Term to Read More and mantleMain silicate-rich zone within a planet between the crust and metallic core. The mantle accounts for 82% of Earth's volume and is composed of silicate minerals rich in Mg. The temperature of the mantle can be as high as 3,700 °C. Heat generated in the core causes convection currents in Click on Term to Read More vs. an exogenous mixture through impact), and ii) if any genetic connection exists between the irons and other meteoriteWork in progress. A solid natural object reaching a planet’s surface from interplanetary space. Solid portion of a meteoroid that survives its fall to Earth, or some other body. Meteorites are classified as stony meteorites, iron meteorites, and stony-iron meteorites. These groups are further divided according to their mineralogy and Click on Term to Read More groups (e.g., IAB with winonaites, IIE with H chondritesChondrites are the most common meteorites accounting for ~84% of falls. Chondrites are comprised mostly of Fe- and Mg-bearing silicate minerals (found in both chondrules and fine grained matrix), reduced Fe/Ni metal (found in various states like large blebs, small grains and/or even chondrule rims), and various refractory inclusions (such Click on Term to Read More, and Eagle Station pallasites with CK chondrites). Three IAB irons were employed in the study, and it was demonstrated on a coupled diagram that although the ε54Cr values for the iron component plot in the winonaitea partially differentiated asteroid that was disrupted just as it began to form an Fe core and a silicate-rich crust. This disrupting impact mixed silicates into molten Ni-Fe metal forming the silicated IAB irons, and mixed olivine-rich residues of partial melts into unmelted silicates, forming the winonaites. A few winonaites Click on Term to Read More field, values for the silicate component plot in a distinct region on an O–Cr coupled diagram (see diagram below). From these results they ascertained that the the IAB silicated irons formed through an impact-generated mixture comprising iron from a winonaite-related parent body and silicate from an unrelated and otherwise unsampled parent body. Incorporation of the silicates into the FeNi-metal host took place at a depth greater than 2 km, allowing time for a Thomson (Widmanstätten) structure to develop during a long cooling phase. Fractional crystallizationA crystallization process in which minerals crystallizing from a magma are isolated from contact with the liquid. It is a key process in the formation of igneous rocks during the process of magmatic differentiation. Also known as crystal fractionation. Click on Term to Read More occurred in some large molten metalElement that readily forms cations and has metallic bonds; sometimes said to be similar to a cation in a cloud of electrons. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties, along with the metalloids and nonmetals. A diagonal line drawn Click on Term to Read More pools, followed by very slow cooling, to produce the broad range of features found in certain IAB meteorites (e.g., silicate-poor, graphite–troilite-rich inclusions and extremely high Ni contents). Other results from their study can be found on the Miles and Eagle Station pages. 17O vs. ε54Cr for Irons and Pallasites
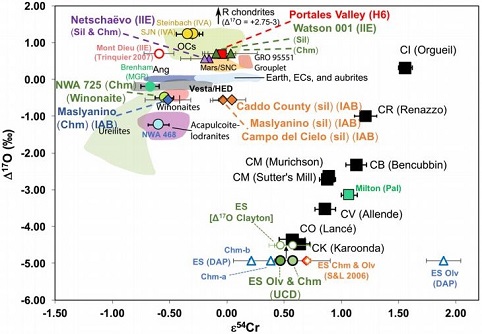
click on photo for a magnified view Diagrams credit: Dey et al., 50th LPSC, #2977 (2019)
The FeNi-chloride named ‘Lawrencite’ was first identified in 1877 in a sample of Tazewell. This mineralInorganic substance that is (1) naturally occurring (but does not have a biologic or man-made origin) and formed by physical (not biological) forces with a (2) defined chemical composition of limited variation, has a (3) distinctive set of of physical properties including being a solid, and has a (4) homogeneous Click on Term to Read More absorbs moisture from the air and liquefies, a property known as deliquescence. The reaction with water and oxygenElement that makes up 20.95 vol. % of the Earth's atmosphere at ground level, 89 wt. % of seawater and 46.6 wt. % (94 vol. %) of Earth's crust. It appears to be the third most abundant element in the universe (after H and He), but has an abundance only Click on Term to Read More produces iron hydroxide and then hydrochloric acid, which can lead to the eventual disintegration of some meteorites. Tazewell is now the type locality for this mineral. To learn more about the relationships within the IAB complex, and among other iron chemical groups, see the Appendix, Part III. The Tazewell specimen shown above is a 2.9 g etched partial slice with a small remnant of fusion crustMelted exterior of a meteorite that forms when it passes through Earth’s atmosphere. Friction with the air will raise a meteorite’s surface temperature upwards of 4800 K (8180 °F) and will melt (ablate) the surface minerals and flow backwards over the surface as shown in the Lafayette meteorite photograph below. Click on Term to Read More. A large slice of Tazewell can be seen on the collection page of Mike Farmer.